Website:
Instron
Website:
Instron
Grupo: Instron
Excertos do catálogo

AUTOINJECTOR TESTING SYSTEM Semi-Automated System for Testing Pen and Autoinjectors to ISO 11608
Abrir o catálogo na página 1
AUTOINJECTOR TESTING SYSTEM Semi-Automated System for Testing Pen and Autoinjectors Developed in close partnership with pharmaceutical device manufacturers, Instron’s latest generation Autoinjector Testing System can perform full functionality testing on a wide range of drug delivery devices – including needle shield and button-activated devices, as well as safety syringes. Instron’s Autoinjector Testing System measures a variety of essential performance requirements, including cap removal, dose accuracy, activation force, injection time, needle depth, and needle guard lockout – allowing...
Abrir o catálogo na página 3
AUTOINJECTOR TESTING SYSTEM Semi-Automated System for Testing Pen and Autoinjectors Due to the complexity of autoinjector devices, labs often require multiple pieces of equipment to perform the wide range of tests required. This approach is more time consuming, requires more specimens, and creates a need for data consolidation. Simplify the Testing Process Instron’s Autoinjector Testing System replaces traditional test procedures that require separate pieces of equipment, enabling users to run a complete sequence of tests on a SINGLE SYSTEM. This allows labs to accelerate time to market by:...
Abrir o catálogo na página 4
FULL FUNCTIONALITY TESTING WITH A SINGLE SYSTEM ACTIVATION FORCE CLICK DETECTION INJECTION TIME NEEDLE DEPTH DELIVERED VOLUME NEEDLE GUARD LOCKOUT Simplify the Testing Process | 05
Abrir o catálogo na página 5
BLUEHILL® UNIVERSAL Dedicated Test Methods Bluehill Universal uses simplified test types that empower users to develop and change method parameters with ease, while offering the flexibility to readily accommodate future devices without requiring Instron’s support. Device Properties Button and needle shield devices are options in the specimen properties tab. The device type is selected and dimensions are entered. These dimensions are used to properly position the crosshead throughout the test sequence, optimizing test time and ensuring tests are run consistently, even on different systems.
Abrir o catálogo na página 6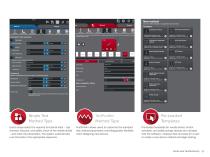
Simple Test Method Type Users simply select the required functional tests – cap removal, injection, and safety check of the needle shield – and enter the parameters. The system automatically runs the tests in the appropriate sequence. TestProfiler Method Type TestProfiler allows users to customize the standard test method parameters, providing greater flexibility when designing new devices. Pre-Loaded Templates Pre-loaded templates for needle shield, button activated, and safety syringe devices are included with the software, making it fast and easy for a user to create a new device method...
Abrir o catálogo na página 7
SYSTEM SUITABILITY TESTING Improved Compliance in Bluehill® Universal Bluehill Universal’s autoinjector testing software integrates system suitability testing into its workflow, prompting users to perform tests and automatically tracking results in the audit trail. Good Manufacturing Practice (GMP) labs require daily checks of transducers prior to testing to ensure they are functioning accurately. In-house development of the hardware and test methods for performing these checks are time consuming and difficult to validate. Often labs use paper records to track the completion and performance...
Abrir o catálogo na página 8
Enforced System Suitability Testing A system administrator can turn on the SST requirement and specify which transducers must be checked as part of the process. Frequency of SSTs are also set by the administrator based on a time period and/or upon the creation of a new sample. Bluehill Universal will prevent additional device testing until the SSTs have been successfully completed. Traceability A report of the system suitability results is stored as a PDF and the successful completion of these checks is recorded in the audit log of the Traceability module, offering an efficient way to...
Abrir o catálogo na página 9
MACHINE VISION CAMERA Needle Depth and Injection Time Instron’s system incorporates a machine vision camera to provide a measured injection time as well as indications of the exposed needle depth at both the start and end of the injection. Measurement of the injection time and needle depth during the injection of the fluid is a critical piece of data to ensure the device is working as therapeutically intended. The use of an optical measurement method to capture this data is more effective than traditional methods, such as gravimetric or laser-based systems, especially as drug formulations...
Abrir o catálogo na página 10
Needle Depth at Start of Injection A measurement of the needle depth when the initial fluid expulsion occurs provides the ability to identify whether the device is delivering its dose into the therapeutic range. This measurement can be brought into Bluehill Universal. A photo of this event is stored, making it available for analysis any time after the test has been completed. Measurement of Injection Time The machine vision camera provides high accuracy measurements of injection time, which supports a wide range of delivery profiles, including high viscosity formulations with non-continuous...
Abrir o catálogo na página 11
ROOT CAUSE ANALYSIS High-Resolution Video Camera Instron’s Autoinjector Testing System uses a high-resolution video camera, along with the machine vision camera, to provide critical visuals to aid in root cause analysis when a device failure occurs. Some device failures are clearly detectable based on the calculated results, data curves, or the appearance of the device itself. Other failures are not obvious until the entire sample batch has been tested and statistically analyzed (K-value). In either scenario it is important to determine whether a bad result was truly a device failure or...
Abrir o catálogo na página 12
Video Recording of Injection Needle Depth Image Storage A high-resolution camera monitors the injection and records a video. This video can be viewed and replayed through Bluehill’s TestCam module, allowing further analysis to be performed on a previously run test. When the video is replayed the closest corresponding data point on the test curve is highlighted. Conversely, a scanning cursor can be used to select a particular point of interest on a data curve which pulls up the associated video frame. Images of the needle at both the start and end of the injection are stored for each device....
Abrir o catálogo na página 13Todos os catálogos e folhetos técnicos Instron
-
DX MODELS
3 Páginas
-
6800 Series Universal Testing Systems
28 Páginas
-
3400 Series Universal Testing Systems
20 Páginas
-
High Force Universal Testing Machines
24 Páginas
-
Composites Testing Solutions
20 Páginas
-
2580 Series Load Cells
3 Páginas
-
2519 Series Static Load Cells
2 Páginas
-
90° & VARIABLE ANGLE PEEL FIXTURE
2 Páginas
-
HDX Models
2 Páginas
-
5980 SERIES Dual Column Floor Model
2 Páginas
-
DAMPER TEST SYSTEMS BI-7080
2 Páginas
-
2860 SERIES PCB CLAMPING STAGE
1 Páginas
-
HIGH STRAIN RATE VHS SYSTEM
4 Páginas
-
HIGH CAPACITY SYSTEMS Up to 5000 kN
4 Páginas
-
3119-600 Series Environmental Chambers
12 Páginas
-
Automated XY Testing System
3 Páginas
-
CEAST 9400 Series
20 Páginas
-
COMPRESSION PLATENS
2 Páginas
-
LOW CYCLE FATIGUE
11 Páginas
-
ElectroPuls™
10 Páginas
-
Pneumatic Side-Action Grips
4 Páginas
-
Automated Specimen Handling Systems
24 Páginas
-
Hip Femoral Fatigue Fixture ISO 7206
2 Páginas
-
3kN Compression Platens 2840-117
2 Páginas
-
Instron Stiffness Based Tuning
2 Páginas
-
CEAST MF Series
20 Páginas
-
Automated Tensile Testing System
2 Páginas
-
HDT AND VICAT TESTING SYSTEMS
16 Páginas
-
AlignPRO™ Alignment System
2 Páginas
-
8800 MiniTower Control Electronics
2 Páginas
-
8800 Tower Control Electronics
2 Páginas
-
CEAST Melt Flow Series
20 Páginas
-
CEAST SmartRheo Series
16 Páginas
-
CEAST 9000 Series
20 Páginas
-
ElectroPuls E1000 Testing System
2 Páginas
Catálogos arquivados
-
CEAST HDT Vicat Series
9 Páginas