
Pharmaceutical Biotechnology: Downstream Processing with Centrifuges and Package Units - Brochure
32Pages
Catalog excerpts

Pharmaceutical Biotechnology Downstream processing with centrifuges and package units from GEA Westfalia Separator engineering for a better world
Open the catalog to page 1
Quality in its Purest Form Downstream processing technology Aseptic process management, optimum cleaning for optim sing production processes and products. i capability, closed product handling, reliable A critical factor underlying the success of the compliance with GMP requirements, gentle product company in pharmaceutical biotechnology is its treatment, efficient recovery of active ingredients ability to swiftly translate new developments into and reliable scale-up – the requirements of pharma- marketable processes and systems which fully ceutical biotechnology are high. With separators...
Open the catalog to page 2
Efficient, reliable and economical GEA Westfalia Separator Group supports new developments in the branch from the outset through continuous cooperation with universities, research institutes and industry. In this way, a rapid and individual response to current customer needs is assured at all times. With stand-alone machines or package units which guarantee a high yield of valuable substances and which operate trouble-free, efficiently, reliably and economically throughout a long service life. Convince yourself!
Open the catalog to page 3
Enzymes are complex organic protein compounds located in every living cell, where they are produced. They accelerate organic processes such as the breakdown of starch, protein, fat or sugar as catalysts, i. e. without being expended themselves. These valuable proteins are known to industry as esteemed helpers. The separators and decanters from GEA Westfalia Separator Group ensure that the intracellular and extracellular enzymes are separated undamaged and in high concentrations. Production of intracellular enzymes Glucose isomerase is an example of an enzyme which converts glucose into...
Open the catalog to page 4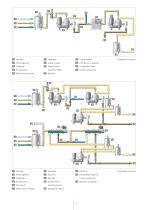
11 Cell debris to disposal (washing liquid) 5 Rotary brush strainer Intracellular enzymes 5 Flocculents 6 Rotary brush strainer further processing 14 Biomass to disposal (washing liquid) 11 Extracellular liquid
Open the catalog to page 5
Water (waste) 12 Packaging Pelletizer Cold store Nutrient solution U Main fermenter Aeration H Nozzle separator Freeze-dried starter cultures H Waste Seed fermenter H Concentrate Microbial food cultures can be subdivided into starter cultures and probiotic products; they are used in many segments of the food industry. Starter cultures are now an established part of the food, medical product and animal feed industries. They are responsible for predictable and reproducible product quality and thus for controllable production processes. Probiotic products are also...
Open the catalog to page 6
from the liquid phase. Nozzle and self-cleaning disc separators in steam-sterilised design are available for this step of the process. The concentrated lactobacilli then pass to a freeze drier. Finally, the cultures are packaged under oxygen exclusion and stored at low temperatures. This retains their activity for months before they are processed, e. g. as “live cultures” in probiotic yoghurts. Sterility and careful treatment The careful treatment of the living microorganisms, sterility and a high separation efficiency are prerequisite to the economical, reliable and efficient processing....
Open the catalog to page 7
Vaccines Protection against germs Vaccines are medicines which immunise human or Process of vaccine manufacturing animal organisms against diseases. The organism When a suitable virus strain has been chosen, it is to be protected is repeatedly exposed to small isolated in ampoules and stored at -192 °C in liquid quantities of antigens (vaccines) or antibodies in nitrogen. Breeding is then conducted starting with the form of a serum. This achieves immunity. the pre-fermenter and continuing in the main fermenter. The fermentation process is supplemented Live vaccines by a nutrient solution....
Open the catalog to page 8
Non-live vaccines In serum therapy, the infected organism is supplied with the sera produced with the antibodies of immunised individuals. Sera are gained by multiplying bacteria in a suitable nutrient solution. The serum produced during fermentation is excreted by the cells into the fermentation solution. The serum is isolated by separating the biomass in centrifuges and by further stages of processing in the clarification phase. An aseptic process is essential in the production of vaccines and sera. ^14 Aeration Starter culture Dried nutrients Water Pre-fermenter Nutrient tank Virus...
Open the catalog to page 9
Hormones / Insulin Separation technology for human medicine Hormones are chemical messengers which have a In the service of human medicine regulating effect on the metabolism and organ Separators from GEA Westfalia Separator Group play activity. In collaboration with the nervous system, an important part in the production process. Nozzle they co-ordinate all bodily functions. A complicated separators operate in the depicted process, in which control mechanism ensures a harmonic interaction the solid material is extracted continuously in a of the bodily functions. The separation technology...
Open the catalog to page 10
How sugar metabolism works The pancreas excretes insulin into the blood-stream. Glucose, blood sugar. Glucose is oxidised in the cells to generate energy. Insulin helps to convey glucose into the cells: it opens the cells. 10 2nd stage nozzle separator 5 1 stage nozzle separator 18 Clear phase to waste 19 Insulin crystals to freeze dryer Example insulin based on yeast
Open the catalog to page 11
Extracellular products Inclusion bodies products Intracellular products 1 Aeration 2 Microorganism 3 Substrate 11 Cellular liquid with cell containing soluble (washing liquid) 7 Extracellular liquid debris (waste) Pharmaceutical Proteins Genetically modified bioproducts Biotechnological processes can be characterised as factor is multiplied by fermentation of the micro- processes employing genetically modified micro- organisms. The DNA chain with the modified gene organisms. These are able to produce biological and the substances which it produces develop products which they would never...
Open the catalog to page 12All GEA Westfalia Separator catalogs and technical brochures
-
Pharma Solids Center
20 Pages
-
Crystallization Technology
20 Pages
-
Evaporation Technology
24 Pages
-
Industrial Drying Brochure
10 Pages
-
Spray Drying for Pharmaceuticals
16 Pages
-
GEA Blu Chillers - Brochure / EN
16 Pages
-
GEA Powdereye
2 Pages
-
GEA VISIOCOVER
2 Pages




















